Abstract
Introduction
Hyperhemolysis has been well described in delayed hemolytic transfusion reaction (DHTR), which is one of the most serious complications of transfusion especially in patients with sickle cell disease (SCD). In the most severe cases, this life-threatening syndrome may reach 10% lysis of total red blood cells (RBCs) (Habibi A et. al. Am J Hematol 2016). DHTR is characterized by an acute anemia with increased plasma free Hb and free heme. Hyperhemolysis can also be found in other hemoglobinopathies and extracorporeal circulation procedures.
Most in vitro studies on hyperhemolysis used only purified Hb or heme which did not allow to investigate all deleterious effects on the endothelium. We have developed a global and physiopathologic approach to assess the mechanism of hyperhemolysis-induced endothelial dysfunction. For this purpose, we created a microfluidic model reproducing hyperhemolysis observed in the most severe DHTR but it's also applicable for other pathogenesis of hyperhemolysis.
Among the potential therapeutic approaches, carbon monoxide (CO) is beneficial on endothelial cells by its anti-inflammatory, antioxidant and vasodilator effect which reduce the toxicity of Hb and improve tissue perfusion (Motterlini R et.al. Nat. Rev. Drug Discov 2010). Here we investigated the therapeutic effects of a CO-releasing molecule, CORM-401, in hyperhemolysis-induced endothelial dysfunction.
Materials and methods
Flow culture Human Umbilical Vein Endothelial Cells (HUVECs) in fibronectin-coated µ-Slides were preconditioned for 4 hours under shear stress of 1dyn/cm² with sonicated RBCs of healthy donors (AA) reconstituted in serum of either AA or SCD patients (SS) at 7 g/L of free Hb. In negative control conditions, cells were pretreated similarly with either AA serum or culture medium. Cells were then collected for analyses of actin network, membrane markers and endothelial function. Treatment with CORM-401 or inactive CORM (iCORM) was performed at fixed concentration during the preconditioning and perfusion steps.
Results
Hemolysis induces multiple endothelial damage and dysfunction
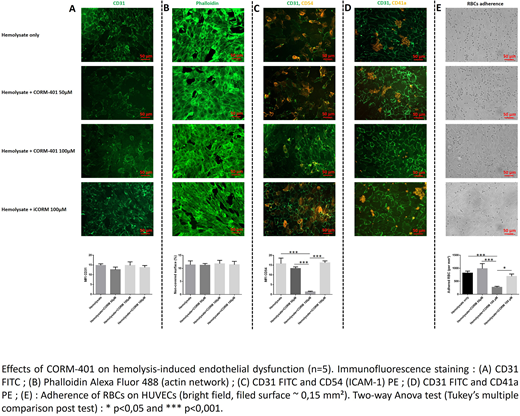
After exposure to hemolysate, we noted a significant increase of subendothelial matrix exposition (fibronectin-coated surfaces) due to actin network reorganization and cells detachment. Immunofluorescence staining showed a moderate activation of HUVECs induced by hemolysate with membrane expression of adhesion molecules such as ICAM-1, E-Selectin and VCAM-1, but not VWF and P-Selectin. At a similar free Hb level, preconditioning with hemolysate without microparticles (MPs) had the lowest deleterious impact on HUVECs suggesting a role of MPs in hyperhemolysis.
Perfusion of AA whole blood on HUVECs pretreated with hemolysate resulted in aggregation and activation of platelets in a GPIIbIIIa-dependent manner at injury sites. We also observed a significant increase of adhered RBCs (14-fold compared to control).
Compared to whole blood RBC lysis, purified Hb induced similar subendothelial exposure and RBC adherence but did not lead to significant HUVECs activation.
To study the DHTR in RBC transfusion of SCD patients, SS serum was used to perform hemolysis preconditioning of HUVECs. Adherence of RBCs was similar to condition using AA serum suggesting that endothelial damage in DHTR of SCD patients depend on hemolysate compositions and RBC MPs, rather than on patients' serum.
Effects of CORM-401 on hemolysis-induced endothelial dysfunction
Adding CORM-401 (50 to 100 µM) to the hemolysate produced 15% free COHb leading to a concentration-dependent decrease in hemolysis-induced HUVECs activation and RBC adherence (Figure 1 C, E). While no significant effects at the subendothelial exposure were observed, the aggregation of platelets at injury sites was decreased after CORM-401 treatment compared to controls (Figure 1 A, B, D), confirming the anti-platelet aggregation effect of this molecule (Chlopicki S et. al. Naunyn Schmiedebergs Arch Pharmacol 2012).
Conclusion
Our in vitro model enabled the reproduction of endothelial damage by hyperhemolysis and to determine the deleterious effects of each hemolysate component. The in vitro beneficial effects of CORM-401 were also demonstrated and an in vivo study on SCD mice is underway to explore the therapeutic effects of this molecule against hyperhemolysis-induced endothelial damage.
Bartolucci:Addmedica: Research Funding; GBT: Membership on an entity's Board of Directors or advisory committees; Fondation Fabre: Research Funding; Novartis US: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal